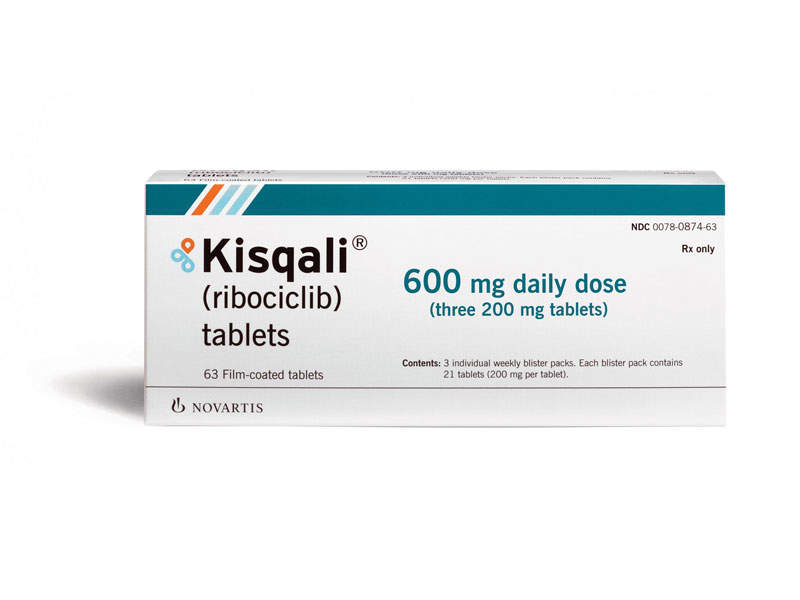
What is Kisqali (ribociclib) for?
Kisqali (ribociclib) is a kinase inhibitor indicated in combination with an aromatase inhibitor as initial endocrine-based therapy for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer1,2.
How does Kisqali (ribociclib) work?
Ribociclib is an inhibitor of cyclin-dependent kinase (CDK) 4 and 6. Before a cell can divide, it has to go through four phases: a growth phase (G1-phase), a synthesis phase (S-phase), another growth phase (G2-phase), and a last phase is where the cell divides (M-phase). Cancer cells divide exceedingly fast, passing through these 4 phases rapidly. Ribociclib blocks the progression from the first G1-phase, into the second S-phase. It does this by inhibiting the cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) – two proteins that are involved in entering the S-phase1.
Where has Kisqali (ribociclib) been approved?
Kisqali (ribociclib) was approved in combination with an aromatase inhibitor as initial endocrine-based therapy for the treatment of postmenopausal women with HR-positive HER2-negative advanced or metastatic breast cancer by:
FDA (USA) on March 13, 20173
EMA (EU) on August 22, 20174
How is Kisqali (ribociclib) taken?
The standard dosage is:
600 mg orally (three 200 mg tablets) taken once daily with or without food for 21 consecutive days, followed by 7 days off treatment (one complete cycle consists of 28 days).
Dose interruption, reduction, and/or discontinuation may be required based on individual safety and tolerability.
Complete information about Kisqali (ribociclib) dosage and administration can be found in the resources section.
Consult your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Kisqali (ribociclib)
Most common adverse reactions (incidence ≥ 20%) are1,4:
low levels of white blood cells
nausea
fatigue
diarrhea
alopecia
vomiting
constipation
headache
back pain.

Reviews
There are no reviews yet.